
- Home
- About
- Research
- Publications
- Media
- Lab Members
- Links & Resources
- Lab Access
- Available Positions
- Contact Us
- Clinical Site
| © Martinez-Agosto Laboratory, Department of Human Genetics, University of California, Los Angeles, 2008-2015. |
Research in Medical Genetics
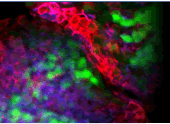
 Our research program focuses on dissecting the molecular pathways that regulate the size of an organ as well as how the final size of an organism is determined. Understanding these processes is relevant to the
behavior and function of stem cells as well as their role in the process of tissue repair and regeneration. In particular, how does a stem cell know how much tissue to reconstitute upon injury? Similarly, as a tissue
regenerates or repairs itself, how does it measure or regulate the extent of its growth and how does it know when to stop growing?
Our research program focuses on dissecting the molecular pathways that regulate the size of an organ as well as how the final size of an organism is determined. Understanding these processes is relevant to the
behavior and function of stem cells as well as their role in the process of tissue repair and regeneration. In particular, how does a stem cell know how much tissue to reconstitute upon injury? Similarly, as a tissue
regenerates or repairs itself, how does it measure or regulate the extent of its growth and how does it know when to stop growing?
A restricted number of signaling pathways have been identified that regulate how and by how much a cell or tissue grows to give rise to an organ of a defined size. We focus our studies on three signaling pathways
that affect tissue growth: ras, PTEN/Akt/Tor and the Salvador-Warts-Hippo (SWH) pathways. The major areas of investigation are:
1. Identification of novel components and drug targets of growth signaling pathways
2. Growth signaling pathways in stem cells and their niches
3. Human growth disorders: overgrowth syndromes, autism, and cancer predisposition
1. Identification of novel components and drug targets of growth signaling pathways
.gif)
We are performing genetic screens using Drosophila Melanogaster to identify new components of the TOR and Salvador-Hippo-Warts signaling pathways. Our screens have identified new signaling networks
that appear to communicate and transduce the growth promoting effects of these pathways. These novel genes are immediate candidates for molecular studies on genetic basis of cancer. We also perform drug compound
screens to identify novel targets for therapy within these growth signaling pathways.
2. Growth signaling pathways in stem cells and their niches
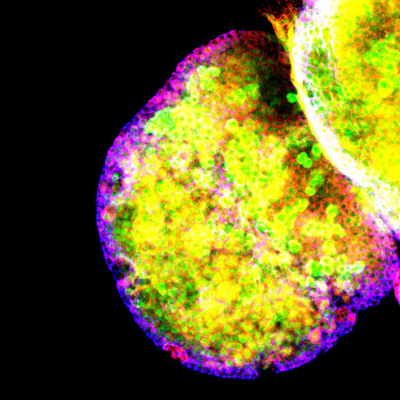
We hypothesize that stem cells and their niches represent aunit of organ and tissuegrowth and predict that growth signaling pathways function in these cells to regulate final organ tissue and tissue repair.
We utilize neural, intestinal and hematopoietic stem cell populations in the fruitfly Drosophila Melanogaster as a model in which to evaluate the role of growth signaling pathways on organ growth in vivo
. This is particularly relevant to growth signaling pathways which require cell-cell contacts and extracellular matrix interactions for their proper function. Our goal is to characterize how growth signaling
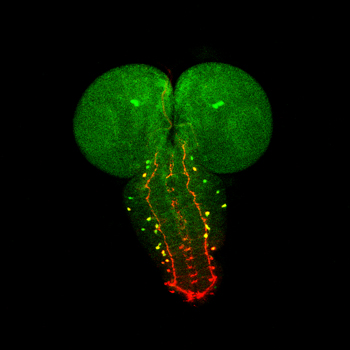
pathways regulate final organ size, particularly in the brain. The regulation of final brain size has both evolutionary and biological relevance in the context of human disease (see below). We hypothesize that growth
signaling pathways regulate stem cell maintenance and proliferation in determining final brain size and may constitute a conserved network
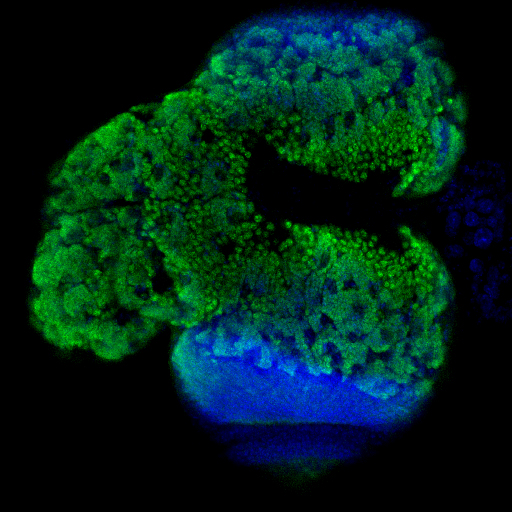
 modified across evolution in the regulation of brain size. These pathways also appear to regulate hormonal secretion required for final organ development.
modified across evolution in the regulation of brain size. These pathways also appear to regulate hormonal secretion required for final organ development.
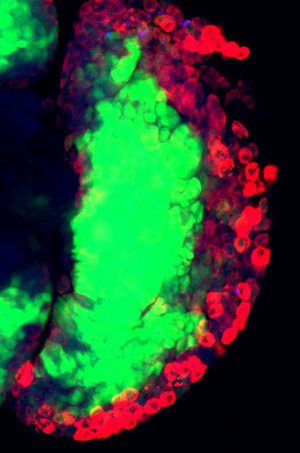
We have previously described the presence of a hematopoietic niche within the Drosophila larva. Our goal is to define the niche-derived and cell-type specific signals required for maintenance of stemness
versus cell differentiation in a population of blood stem-like precursors as a framework for understanding the biology of vertebrate stem cell-niche function.
Through in vivo genetic screens and in vitro assays of cultured blood cell precursors, we seek to identify novel pathways which regulate the formation of niche cells and which maintain their identity as signaling cells
required for the maintenance of hematopoietic progenitors in the Drosophila lymph gland. In addition, we utilize lineage tracing and label retention techniques to identify novel stem cell niches in which to
model human disease. Finally, we utilize mathematical approaches to model stem cell-niche interactions and apply them to predict outcomes from therapy in human disorders.
3. Human growth disorders: overgrowth syndromes, autism, and cancer predisposition
Overgrowth syndromes: We are interested in understanding the common signal transduction pathways across a number of human genetic conditions in which there is excessive tissue and organ growth. These genetic
diseases are associated with cancer predisposition and include overgrowth syndromes suich as Sotos, Beckwith-Wiedemann, Weaver, and Simpson-Golabi-Behmel. Their associated malignancies are primarily neuroblastoma,
sarcomas, and myeloproliferative disorders.
To this end we utilize two major approaches to better understand the pathophysiology of these conditions:
1. We use Drosophila Melanogaster as a model organism in which to study the cellularand genetic mechanisms that lead to oncogenesis due to the signal transductionpathways disrupted in these syndromes. In
particular, we seek to dissect the role of stem cell niches in cancer predisposition. In addition, we perform translationaldevelopmental biology by performing small molecule compound screens in vivo (Drosophila larval
screens)and in vitro (stem cell cultures) to identify therapies for these pediatric cancers and genetic syndromes.
2. We use whole exome sequencing technology to identify the genes associated with these conditions.
Autism as a brain growth disorder: We study autism and its association with large head circumference as a brain growth disorder. We hypothesize that many cases of autism are due to alterations in PTEN/Tor
signaling, leading to abnormal brain growth. By identifying copy number variations and using whole exome approaches we seek to identify novel genes that may predispose to autism when associated with microcephaly.
Growth signaling pathways in cancer: We validate our findings from human syndromes and Drosophila genetic screens in human cancer cell lines and patient cell lines from genetic conidtions that affect growth.
This allows for an immediate clinical translational, both diagnostic and therapeutic, application of the basic findings from our ghenomic and genetic approaches.